Which Of The Followeing Diagram Direct Change
Phase Diagrams
- Page ID
- 1535
Stage diagram is a graphical representation of the physical states of a substance under different conditions of temperature and force per unit area. A typical stage diagram has pressure on the y-axis and temperature on the 10-centrality. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist in equilibrium on the lines or curves.
Introduction
A phase transition is the transition from one state of matter to another. There are three states of matter: fifty iquid, solid, and gas.
- Liquid: A state of matter that consists of loose, complimentary moving particles which class the shape set by the boundaries of the container in which the liquid is in. This happens considering the motility of the individual particles within a liquid is much less restricted than in a solid. 1 may notice that some liquids period readily whereas some liquids menses slowly. A liquid's relative resistance to flow is viscosity.
- Solid: A state of matter with tightly packed particles which do not change the shape or volume of the container that information technology is in. However, this does not hateful that the volume of a solid is a constant. Solids can expand and contract when temperatures change. This is why when you look up the density of a solid, information technology will indicate the temperature at which the value for density is listed. Solids have strong intermolecular forces that go along particles in close proximity to one another. Another interesting thing to call back well-nigh is that all truthful solids have crystalline structures. This ways that their particles are bundled in a iii-dimensional, orderly pattern. Solids will undergo phase changes when they come across free energy changes.
- Gas: A state of thing where particles are spread out with no definite shape or volume. The particles of a gas volition have the shape and fill the volume of the container that it is placed in. In a gas, at that place are no intermolecular forces holding the particles of a gas together since each particle travels at its own speed in its own direction. The particles of a gas are often separated past smashing distances.
Phase diagrams illustrate the variations between united states of matter of elements or compounds as they relate to pressure and temperatures. The following is an instance of a phase diagram for a generic single-component organization:

- Triple signal – the indicate on a phase diagram at which the iii states of matter: gas, liquid, and solid coexist
- Disquisitional point – the indicate on a phase diagram at which the substance is indistinguishable between liquid and gaseous states
- Fusion(melting) (or freezing) curve – the curve on a phase diagram which represents the transition between liquid and solid states
- Vaporization (or condensation) bend – the curve on a phase diagram which represents the transition between gaseous and liquid states
- Sublimation (or deposition) bend – the curve on a phase diagram which represents the transition betwixt gaseous and solid states
Stage diagrams plot pressure (typically in atmospheres) versus temperature (typically in degrees Celsius or Kelvin). The labels on the graph represent the stable states of a system in equilibrium. The lines represent the combinations of pressures and temperatures at which two phases tin exist in equilibrium. In other words, these lines ascertain phase change points. The red line divides the solid and gas phases, represents sublimation (solid to gas) and degradation (gas to solid). The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The blueish divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). There are also two important points on the diagram, the triple point and the critical point. The triple point represents the combination of pressure and temperature that facilitates all phases of matter at equilibrium. The disquisitional betoken terminates the liquid/gas phase line and relates to the disquisitional pressure level, the pressure above which a supercritical fluid forms.
With near substances, the temperature and pressure related to the triple point lie beneath standard temperature and force per unit area and the pressure for the disquisitional point lies to a higher place standard force per unit area. Therefore at standard pressure every bit temperature increases, most substances alter from solid to liquid to gas, and at standard temperature as pressure level increases, near substances change from gas to liquid to solid.
Exception: Water
Normally the solid/liquid phase line slopes positively to the right (equally in the diagram for carbon dioxide below). However for other substances, notably h2o, the line slopes to the left every bit the diagram for water shows. This indicates that the liquid phase is more dense than the solid phase. This phenomenon is caused by the crystal structure of the solid phase. In the solid forms of h2o and some other substances, the molecules crystalize in a lattice with greater average space between molecules, thus resulting in a solid with a lower density than the liquid. Because of this phenomenon, one is able to melt ice simply by applying pressure and not by calculation estrus.
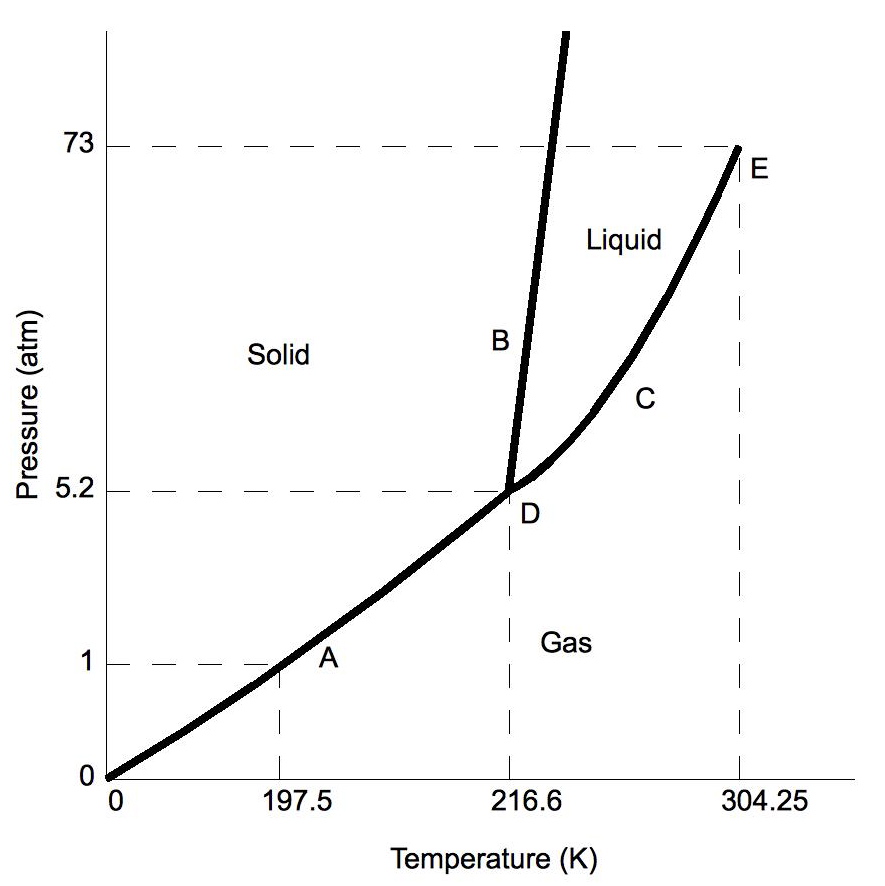
Effigy \(\PageIndex{2a}\): Phase diagrams for CO2
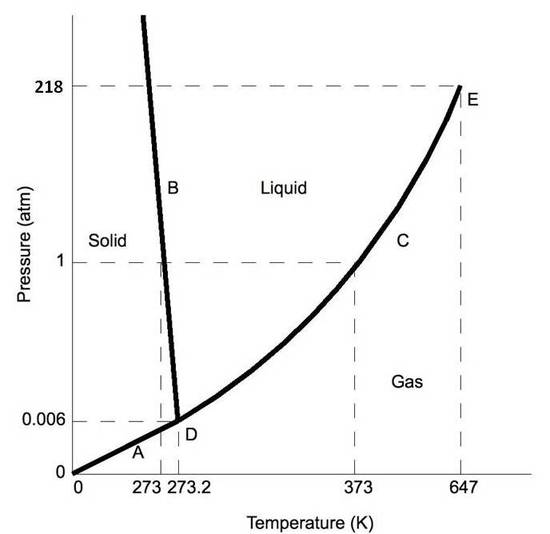
Figure \(\PageIndex{2b}\):Phase diagrams for H2O
Moving About the Diagram
Moving nigh the phase diagram reveals information about the phases of matter. Moving along a abiding temperature line reveals relative densities of the phases. When moving from the bottom of the diagram to the acme, the relative density increases. Moving along a abiding pressure line reveals relative energies of the phases. When moving from the left of the diagram to the right, the relative energies increases.
Of import Definitions
- Sublimation is when the substance goes directly from solid to the gas land.
- Deposition occurs when a substance goes from a gas state to a solid state; it is the reverse procedure of sublimation.
- Melting occurs when a substance goes from a solid to a liquid state.
- Fusion is when a substance goes from a liquid to a solid state, the contrary of melting.
- Vaporization (or evaporation) is when a substance goes from a liquid to a gaseous land.
- Condensation occurs when a substance goes from a gaseous to a liquid country, the opposite of vaporization.
- Disquisitional Bespeak – the point in temperature and pressure level on a phase diagram where the liquid and gaseous phases of a substance merge together into a single phase. Beyond the temperature of the critical betoken, the merged single phase is known as a supercritical fluid.
- Triple Bespeak occurs when both the temperature and pressure level of the three phases of the substance coexist in equilibrium.
References
- Kotz, John C., and Paul Jr. Treichel. Chemistry & Chemic Reactivity. Northward.p.: Saunders College Publishing, 1999.
- Oxtoby, David W., H. P. Gillis, and Alan Campion. Principles of Modern Chemistry. Belmont, CA: Thomson Brooks/?Cole, 2008.
- Petrucci, Ralph, and William Harwood. F. Geoffrey Herring. Jeffry Madura. General Chemistry: Principles and Mod Applications. 9th ed. Upper Saddle River, NJ: Pearson, 2007.
- Vollmer, John J. "Out of "Thin Air": Exploring Phase Changes.' J. Chem. Educ. 2000: 77, 488A.
Problems
Imagine a substance with the following points on the phase diagram: a triple point at .five atm and -5°C; a normal melting signal at 20°C; a normal humid point at 150°C; and a critical point at 5 atm and 1000°C. The solid liquid line is "normal" (meaning positive sloping). For this, complete the following:
one. Roughly sketch the phase diagram, using units of atmosphere and Kelvin.
- Answer
-
1-solid, 2-liquid, 3-gas, 4-supercritical fluid, bespeak O-triple point, C-critical point -78.five °C (The phase of dry out ice changes from solid to gas at -78.5 °C)
2. Rank the states with respect to increasing density and increasing free energy.
3. Describe what one would encounter at pressures and temperatures higher up five atm and k°C.
- Answer
-
One would encounter a super-disquisitional fluid, when approaching the point, one would run into the meniscus betwixt the liquid and gas disappear.
4. Depict what volition happen to the substance when it begins in a vaccum at -15°C and is slowly pressurized.
- Respond
-
The substance would brainstorm every bit a gas and as the pressure increases, information technology would compress and eventually solidify without liquefying equally the temperature is below the triple bespeak temperature.
5. Depict the phase changes from -80°C to 500°C at ii atm.
- Answer
-
The substance would melt at somewhere around, but to a higher place 20°C and then boil at somewhere around, merely above 150°C. It would not form a super-disquisitional fluid every bit the neither the pressure nor temperature achieve the critical pressure or temperature.
six. What exists in a system that is at i atm and 150°?
- Respond
-
Depending on how much free energy is in the organization, in that location will be different amounts of liquid and gas at equilibrium. If just enough energy was added to raise the temperature of the liquid to 150°C, at that place will just be liquid. If more was added, there volition exist some liquid and some gas. If just enough energy was added to alter the land of all of the liquid without raising the temperature of the gas, there will just be gas.
vii. Label the area ane, 2, three, and 4 and points O and C on the diagram.
8. A sample of dry ice (solid COtwo) is cooled to -100 °C, and is set on a tabular array at room temperature (25 °C). At what temperature is the rate of sublimation and degradation the same? (Assume pressure level is held abiding at ane atm).
Contributors and Attributions
- Matthew McKinnell (UCD), Jessie Verhein (UCD), Pei Yu (UCD), Lok Ka Chan (UCD), Jessica Dhaliwal (UCD), Shyall Bhela (UCD), Candace Wong-Sing (UCD)
Which Of The Followeing Diagram Direct Change,
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Phase_Transitions/Phase_Diagrams
Posted by: xiongbefornes.blogspot.com


0 Response to "Which Of The Followeing Diagram Direct Change"
Post a Comment